Rationale for the regulated transition to non-lead products in Canada: A policy discussion paper - ScienceDirect
By A Mystery Man Writer
Last updated 21 Sept 2024


Characteristics, potentials, and challenges of transdisciplinary research - ScienceDirect

Use of ribaxamase (SYN-004), a β-lactamase, to prevent Clostridium difficile infection in β-lactam-treated patients: a double-blind, phase 2b, randomised placebo-controlled trial - The Lancet Infectious Diseases

ICT sector, digitization and environmental sustainability: A systematic review of the literature from 2000 to 2022 - ScienceDirect

Microglia drive transient insult-induced brain injury by chemotactic recruitment of CD8+ T lymphocytes: Neuron

Rationale for the regulated transition to non-lead products in Canada: A policy discussion paper - ScienceDirect

Implementation approaches to improve environmental sustainability in operating theatres: a systematic review - British Journal of Anaesthesia

Standards efforts and landscape for rapid microbial testing methodologies in regenerative medicine - Cytotherapy

Systematic Review: Nonverbal Learning Disability - Journal of the American Academy of Child & Adolescent Psychiatry

Low-dose imipramine for refractory functional dyspepsia: a randomised, double-blind, placebo-controlled trial - The Lancet Gastroenterology & Hepatology

Processing laboratory considerations for multi-center cellular therapy clinical trials: a report from the Consortium for Pediatric Cellular Immunotherapy - Cytotherapy

PDF) Entrepreneurship in Value Chains of Non-timber Forest Products
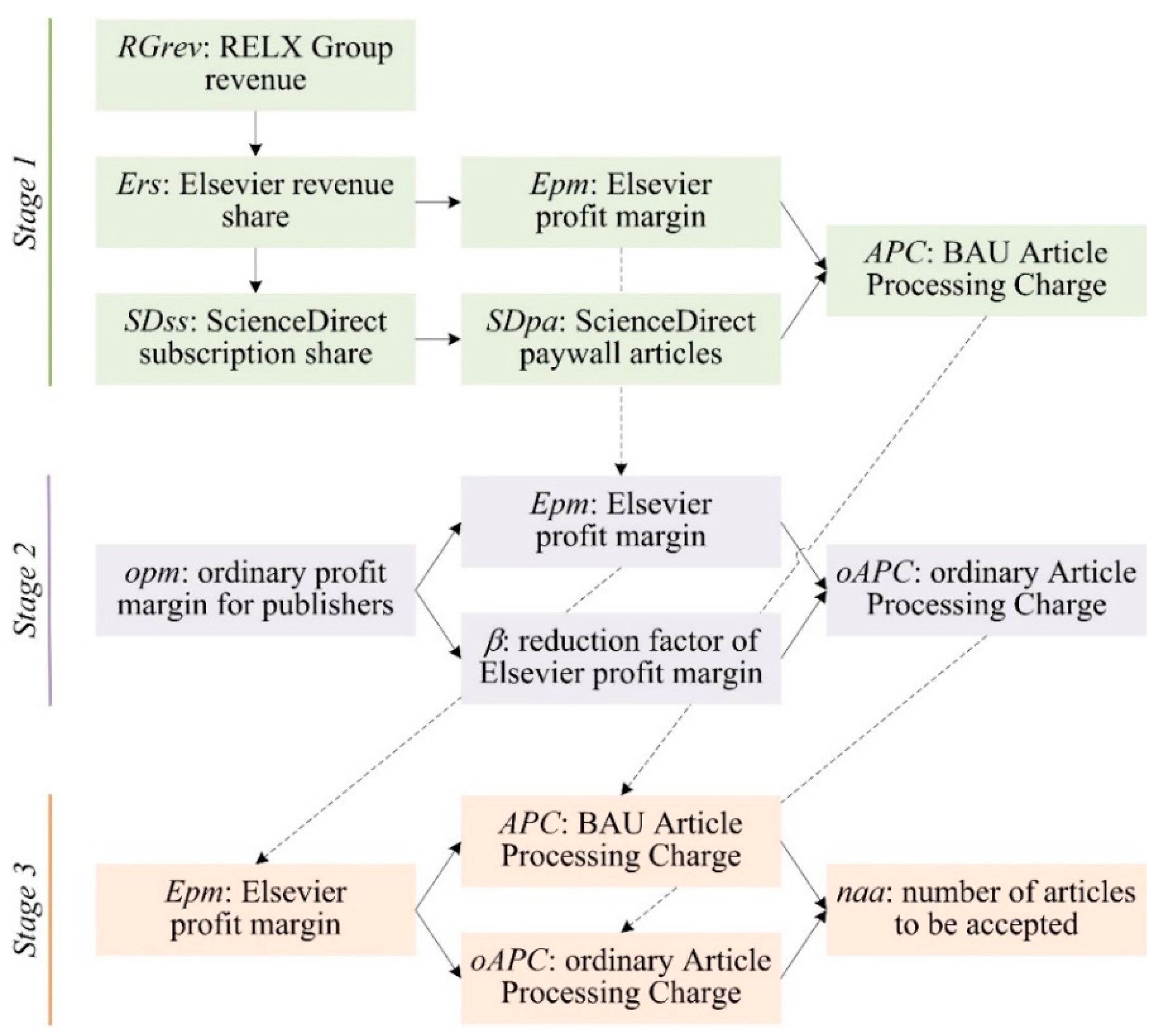
Publications, Free Full-Text

Chemical pollution: A growing peril and potential catastrophic risk to humanity - ScienceDirect

73 questions with answers in PUBLICATION ETHICS
Recommended for you
 Inline Fishing Weights Inline Sinkers Fishing Weights in-line Fishing Sinkers with Inner Swivels Pack of 20 (20 Pcs #2.5(1/3oz)) : : Sports & Outdoors14 Jul 2023
Inline Fishing Weights Inline Sinkers Fishing Weights in-line Fishing Sinkers with Inner Swivels Pack of 20 (20 Pcs #2.5(1/3oz)) : : Sports & Outdoors14 Jul 2023 ECOFT Lead Free Fishing Sinkers and Weights Coated Raindrop14 Jul 2023
ECOFT Lead Free Fishing Sinkers and Weights Coated Raindrop14 Jul 2023 Iron Fishing Weight Sinker, 27/62pcs Bass Casting Sinker Weight14 Jul 2023
Iron Fishing Weight Sinker, 27/62pcs Bass Casting Sinker Weight14 Jul 2023- 5 pcs】Fishing Weights Lead Round Sinker Bottom Batu Alat Pancing14 Jul 2023
 Heavy Duty Non-Lead Egg Sinkers14 Jul 2023
Heavy Duty Non-Lead Egg Sinkers14 Jul 2023 Fishing Tungsten Weights Sinkers Jig Japan Hook Teardrop Jig Head Drop Shot Weights Bullet Weights Free Jika Rig Drop Shot14 Jul 2023
Fishing Tungsten Weights Sinkers Jig Japan Hook Teardrop Jig Head Drop Shot Weights Bullet Weights Free Jika Rig Drop Shot14 Jul 2023 Sinker - Round - (Non Lead)14 Jul 2023
Sinker - Round - (Non Lead)14 Jul 2023 Ximing Copper Weight Sinkers /Fishing Weights Sinker Bulk Sinker Weights , 10g Gold 10g14 Jul 2023
Ximing Copper Weight Sinkers /Fishing Weights Sinker Bulk Sinker Weights , 10g Gold 10g14 Jul 2023 Flat Teardrop-shaped Weights Pure Lead Sinkers Sea Fishing Sinker14 Jul 2023
Flat Teardrop-shaped Weights Pure Lead Sinkers Sea Fishing Sinker14 Jul 2023 OWL Volume 4 Newsletter 2019 – OWL Rehab14 Jul 2023
OWL Volume 4 Newsletter 2019 – OWL Rehab14 Jul 2023
You may also like
 i.img.com/thumbs/images/g/rCwAAOSwi6RmDzkZ/s-l14 Jul 2023
i.img.com/thumbs/images/g/rCwAAOSwi6RmDzkZ/s-l14 Jul 2023 LGBTQ Rainbow Sunflower Retractable Badge Reel Cute Pronoun ER14 Jul 2023
LGBTQ Rainbow Sunflower Retractable Badge Reel Cute Pronoun ER14 Jul 2023- ᐅ Wanipigow Lake fishing reports🎣• Manitoba, Canada fishing14 Jul 2023
 Grub Boss Black Nickel Barbless S14, Fly Tying Hooks14 Jul 2023
Grub Boss Black Nickel Barbless S14, Fly Tying Hooks14 Jul 2023 Shower Curtain Hooks, Set of 12 Heavy Duty Metal Decorative Shower Curtain Rings Vintage Rustproof Curtain Hooks Hangers for Bathroom Shower Rods14 Jul 2023
Shower Curtain Hooks, Set of 12 Heavy Duty Metal Decorative Shower Curtain Rings Vintage Rustproof Curtain Hooks Hangers for Bathroom Shower Rods14 Jul 2023 Guy Harvey Shirt Mens XXL 2XL Red Long Sleeve Graphic Drum Fishing14 Jul 2023
Guy Harvey Shirt Mens XXL 2XL Red Long Sleeve Graphic Drum Fishing14 Jul 2023 South Bend 3-Way Swivel Fishing Accessory, Brass, Size 1, 3-pack14 Jul 2023
South Bend 3-Way Swivel Fishing Accessory, Brass, Size 1, 3-pack14 Jul 2023 Rubber Boots for Men, Mid Calf Waterproof Rain Boots, with 5mm Neoprene Insulated Men Boots, Waterproof Work boots for Mud Hunting Gardening Outdoor14 Jul 2023
Rubber Boots for Men, Mid Calf Waterproof Rain Boots, with 5mm Neoprene Insulated Men Boots, Waterproof Work boots for Mud Hunting Gardening Outdoor14 Jul 2023 TOMA Octopus Jigging Spinning Carbon Fishing Rod Baitcasting 1.8m 2.1m 2 Section MH Inshore Sea Boat Jig Fishing Rod Saltwater14 Jul 2023
TOMA Octopus Jigging Spinning Carbon Fishing Rod Baitcasting 1.8m 2.1m 2 Section MH Inshore Sea Boat Jig Fishing Rod Saltwater14 Jul 2023 MOLIX Scented Floating Soft Bait Lure STICK FLEX 2.7514 Jul 2023
MOLIX Scented Floating Soft Bait Lure STICK FLEX 2.7514 Jul 2023

/ksowg6zqndvbjr3vfr0wtwxlhiiv)